Adsorption and hydrogenation mechanism of crotonaldehyde on a Pd(111) surface by periodic DFT calculations
Abstract
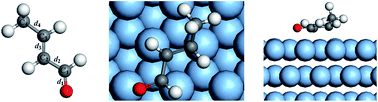
The adsorption of a crotonaldehyde molecule at a Pd(111) surface and its mechanism of hydrogenation are elucidated by a density-functional theory method (periodic DMol3) at the GGA/PW91 level. All the pertinent species of different mechanisms were assessed to obtain their preferred adsorption sites. The activation energy and reaction energy of each step in different mechanisms were also calculated. The results show that the adsorption at the fcc–hcp site is the most stable state when the crotonaldehyde plane is parallel to the Pd(111) surface through the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C and C
C and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O double bonds. The full hydrogenation mechanism follows the hydrogenation steps of O → C2 → C3 → C1 to generate the product of n-butane. For the partial hydrogenation mechanism, the 1,4-addition is identified as a primary mechanism to yield butenal, which further tautomerizes to n-butanal readily. Moreover, no matter what kind of dominant mechanism it is, the first hydrogenation process is the rate-determining step and the reaction is exothermic, therefore reducing the temperature is beneficial to the hydrogenation reaction of crotonaldehyde.
O double bonds. The full hydrogenation mechanism follows the hydrogenation steps of O → C2 → C3 → C1 to generate the product of n-butane. For the partial hydrogenation mechanism, the 1,4-addition is identified as a primary mechanism to yield butenal, which further tautomerizes to n-butanal readily. Moreover, no matter what kind of dominant mechanism it is, the first hydrogenation process is the rate-determining step and the reaction is exothermic, therefore reducing the temperature is beneficial to the hydrogenation reaction of crotonaldehyde.

 Please wait while we load your content...
Please wait while we load your content...