Selective synthesis of propofol (2,6-diisopropylphenol), an intravenous anesthetic drug, by isopropylation of phenol over H-beta and H-mordenite
Abstract
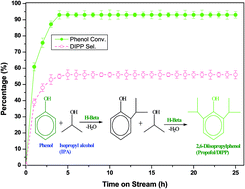
Propofol (2,6-diisopropylphenol/DIPP) is the world's most widely used intravenous general anesthetic and is typically synthesized by isopropylation of phenol over an acid catalyst. It is highly difficult to stabilize bio-oil containing phenolic compounds. The isopropylation of this phenol (a model compound representing species in bio-oils) is one of the options to stabilize the bio-oil and convert it into valuable products. Probably for the first time, H-beta- and H-mordenite-catalysed vapour phase isopropylation of phenol with isopropyl alcohol (IPA) was studied to selectively synthesize DIPP. The optimization of various operating parameters such as molar ratio (phenol : IPA), weight hourly space velocity (WHSV), reaction temperature and time on stream were performed. H-beta (94% phenol conv. and 56% DIPP sel.) was found to be a potential and more active catalyst than H-mordenite (68% phenol conv. and 43% DIPP sel.) at optimized process parameters. A kinetic model is proposed to probe the intricate reaction kinetics and validated (R2 > 0.98) by the experimental results. H-beta catalyst was observed to be stable for more than 25 h with 94% phenol conversion and 56% selectivity towards DIPP at optimized process parameters. The phenol conversion and DIPP selectivity obtained in the present study are higher than those reported so far. The activation energy obtained for isopropylation of phenol with IPA over H-beta is calculated to be 25.39 kJ mol−1.

 Please wait while we load your content...
Please wait while we load your content...