Conformational complexity of morphine and morphinum in the gas phase and in water. A DFT and MP2 study†
Abstract
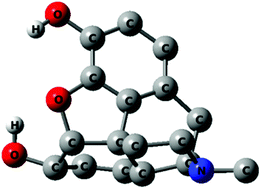
The structural and conformational properties of morphine and protonated morphine (morphinum) in the gas phase and in water solution have been explored with quantum calculations. Fully optimized calculations using the cc-pVTZ basis set, with various methods (MP2, B3LYP, and PBE0) for the species in the gas phase and with B3LYP with simulation of the solvent effect as a continuum with the SMD method were conducted. The study focuses on the determination of the relative energies of the 12 possible conformers that arise from the orientation of the two OH groups and the equatorial vs. axial position of the methyl group on the nitrogen and the energy barriers that separate these minima. The calculations indicate a preference for conformers having the methyl group equatorial, but corresponding axial conformers are not significantly higher in energy. Only 8 of the 12 possible conformers of gaseous morphine were found to be minima on the potential energy hypersurface. All 12 conformers of morphinum are minima according to MP2 computations. B3LYP/SMD (water) calculations predict the coexistence of 12 conformers for both morphine and morphinum with energy ranges of 17 kJ mol−1 for morphine, and as low as 13 kJ mol−1 for morphinum. In morphinum, energy differences of less than 8 kJ mol−1 are computed for 8 conformers, including axial forms. The inversion at nitrogen is calculated to be energetically accessible at room temperature since the activation barrier is less than 30 kJ mol−1 in the gas phase and only around 40 kJ mol−1 with simulated water solvation. The many conformers within a small energy span, the fact that a thermodynamic equilibrium exists between morphine and morphinum in water, and the rapid nitrogen inversion show that morphine and morphinum have a large conformational diversity in water, and thus in the physiological media, which could be a clue to the interaction of this drug with receptors.

 Please wait while we load your content...
Please wait while we load your content...