Enzyme-catalysed oxidation of 1,2-disulfides to yield chiral thiosulfinate, sulfoxide and cis-dihydrodiol metabolites†
Abstract
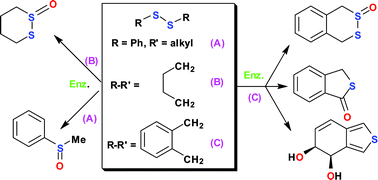
Enantioenriched and enantiopure thiosulfinates were obtained by asymmetric sulfoxidation of cyclic 1,2-disulfides, using chemical and enzymatic (peroxidase, monooxygenase, dioxygenase) oxidation methods and chiral stationary phase HPLC resolution of racemic thiosulfinates. Enantiomeric excess values, absolute configurations and configurational stabilities of chiral thiosulfinates were determined. Methyl phenyl sulfoxide, benzo[c]thiophene cis-4,5-dihydrodiol and 1,3-dihydrobenzo[c]thiophene derivatives were among unexpected types of metabolites isolated, when acyclic and cyclic 1,2-disulfide were used as substrates for Pseudomonas putida strains. Possible biosynthetic pathways are presented for the production of metabolites from 1,4-dihydrobenzo-2,3-dithiane, including a novel cis-dihydrodiol metabolite that was also derived from benzo[c]thiophene and 1,3-dihydrobenzo[c]thiophene.

 Please wait while we load your content...
Please wait while we load your content...