Solvent and catalyst free route to 3-indolyl glycoconjugates: synthesis of sugar tethered isoxazolines and isoxazoles from 3-indolyl nitroalkanes†
Abstract
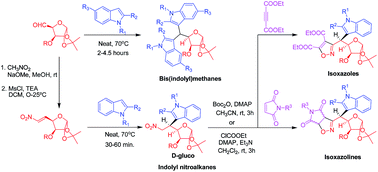
An expedient, solvent and catalyst free strategy for the synthesis of sugar based bis(indolyl)methanes and indolyl nitroalkanes has been developed. The protocol works well within a wide range of substrates, and tolerates various N/O protecting groups. The sugar tethered nitroalkanes were converted to the corresponding nitrile oxides, and subsequently transformed to novel isoxazolines and isoxazoles by cycloaddition with appropriate dipolarophiles.

 Please wait while we load your content...
Please wait while we load your content...