One-pot synthesis of unsymmetrical diarylacetylenes via Sonogashira/deacetonation/Sonogashira cross-coupling of two different aryl chlorides with 2-methyl-3-butyn-2-ol†
Abstract
With the assistance of PdCl2/X-Phos as the catalyst system, a green and efficient protocol for one-pot Sonogashira/Deacetonation/Sonogashira coupling reaction of two different aryl chlorides with 2-methyl-3-butyn-2-ol was developed, affording various unsymmetrical diarylacetylenes in mostly moderate to excellent yields. Note that the cheap and economically available aryl chlorides and 2-methyl-3-butyn-2-ol as the starting materials could be added to the catalyst system directly and simultaneously. Moreover, this tandem reaction could tolerate substrates bearing one or even two ortho-sterically hindered groups and was also applicable to the synthesis of symmetrical diarylacetylenes. In addition, the competitive reaction was performed and a possible mechanism was also proposed.
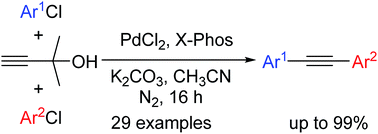

 Please wait while we load your content...
Please wait while we load your content...