A novel chromic oxide catalyst for NO oxidation at ambient temperature
Abstract
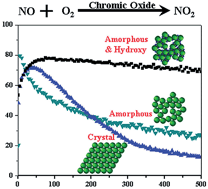
Chromic oxide catalysts were prepared by ammonia precipitation method and first used in NO oxidation to NO2 at ambient temperature. After calcination at 300 °C, the catalyst exhibited higher catalytic activity with 100% conversion of NO and significantly longer lifetime than activated carbon or activated carbon fiber that has been reported so far. The effects of crystalline phases and surface hydroxyl groups on the activity as well as the durability of catalysts in NO oxidation were investigated by XRD, HRTEM, TG, XPS, Raman and DRIFT techniques. The results indicated that the amorphous structure and surface hydroxyl groups together contributed to the high catalytic activity and long-time stability of the catalysts. The promoting effect of surface hydroxyl groups for long-time stability was confirmed by the alkaline treatments of amorphous chromic oxide catalysts.

 Please wait while we load your content...
Please wait while we load your content...