Redox-controlled fluorescence modulation (electrofluorochromism) in triphenylamine derivatives†
Abstract
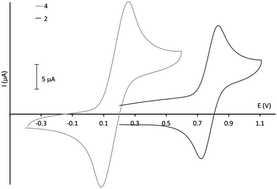
The study of the chemical and electrochemical fluorescence switching properties of a family of substituted triphenylamine derivatives is reported. First of all, the synthesis of a family of six compounds is described. They are characterized by electrochemistry, UV-vis and fluorescence spectroscopy and spectroelectrochemistry. Theoretical calculations were performed in order to corroborate the experimental results. While these compounds emit blue to green light under UV irradiation with a large quantum yield (37%) in the case of one molecule, the fluorescence intensity is quenched upon oxidation. The fluorescence behavior can be switched between the strong fluorescent (neutral) state and the non-fluorescent (oxidized) state with a high contrast (around 1500 for the fluorescence intensity for one of these molecules). Furthermore, the chromatic contrast of three of these molecules reaches 70% that can be important for further applications.

 Please wait while we load your content...
Please wait while we load your content...