Chemical decomposition of epoxy resin in near-critical water by an acid–base catalytic method
Abstract
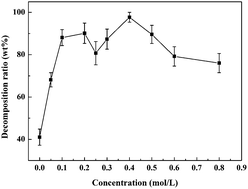
Chemical decomposition of an epoxy resin (E-51) cured with methyl tetrahydro phthalic anhydride (MeTHPA) in near-critical water conditions was investigated under different concentrations of acid–base catalyst. The optimal catalytic concentration of KOH catalyst ranged from 0.5 mol L−1 to 1.0 mol L−1, while that of H2SO4 catalyst was 0.4 mol L−1. Meanwhile, the decomposition ratio of the E-51/MeTHPA system could reach up to 97.7–100%. In addition, FT-IR results of the solid residue of the E-51/MeTHPA system before and after near-critical water treatment showed that the changes of the molecular structure were mainly reflected in the changes of the relative contents of mixed ether bonds, cross-linked bonds and other functional groups. The decomposition products in the acetone phase were identified by GC-MS. The results suggested that the main compositions and relative peak areas of the decomposition products varied with the change of the concentration of acid–base catalyst. Finally, a possible decomposition reaction mechanism was proposed for the E-51/MeTHPA system.

 Please wait while we load your content...
Please wait while we load your content...