Modification of lignin with dodecyl glycidyl ether and chlorosulfonic acid for preparation of anionic surfactant
Abstract
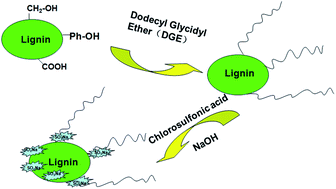
Lignin was modified through incorporation of lipophilic and hydrophilic groups for the preparation of a surfactant. In this case, alkaline lignin reacted with dodecyl glycidyl ether in the presence of dimethyl benzyl amine to incorporate lipophilic long alkyl chains, and then sulfonated with chlorosulfonic acid for the introduction of hydrophilic sulfonic acid groups. Results showed that the reaction between dodecyl glycidyl ether and carboxy group in lignin was the predominant reaction at 95–110 °C. It was found that the surface tension of the synthesized lignin surfactant solution was lower than that of commercial surfactant sodium dodecylbenzenesulphonate when the concentration was below 0.4%, indicating that the surfactant prepared from alkaline lignin had a good surface activity. A lowest critical micelle concentration of 0.50 g L−1 and the corresponding surface tension at 29.17 mN m−1 were achieved when the surfactant was derived from the lignin grafted with dodecyl glycidyl ether at 110 °C. The anionic lignin surfactants prepared in this study are a promising feedstock as detergents or to enhance oil recovery.

 Please wait while we load your content...
Please wait while we load your content...