P4S10 and Na2S-mediated novel annulation routes to c-fused thiophenes†
Abstract
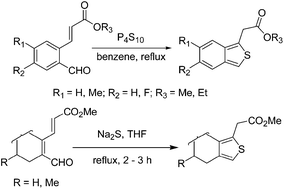
Two efficient hetero-annulation protocols have been developed for the synthesis of stable substituted c-fused thiophene derivatives with moderate to high yield. 3-(2-formyl-phenyl)-acrylic acid esters afforded benzo[c]thiophenes in the presence of phosphorus pentasulfide in refluxing benzene while 3-(2-formyl-cycloalkenyl)α,β-unsaturated esters resulted in the formation of c-fused thiophene derivatives when reacted with sodium sulfide in refluxing tetrahydrofuran.

 Please wait while we load your content...
Please wait while we load your content...