Simultaneous influence of Zn2+/Mg2+ on the luminescent behaviour of La2O3:Tm3+–Yb3+ phosphors
Abstract
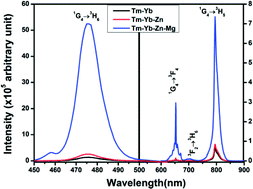
The structural characterizations of La2O3 phosphors doped/codoped with Tm3+ and Yb3+ ions synthesized by the urea assisted solution combustion technique were performed using X-ray diffraction analysis, Fourier transform infrared spectroscopy and scanning electron microscopy. Codoping with Zn2+/Mg2+ ions causes an increase in the particle size and aggregation for the La2O3:Tm3+–Yb3+ phosphor. The upconversion (UC) study under 980 nm excitation shows four UC emission bands centred around 476 nm (1G4 → 3H6), 653 nm (1G4 → 3F4), 702 nm (3F2 → 3H6) and 795 nm (1G4 → 3H5). The effect of codoping with Zn2+ and Mg2+ ions in the Tm3+–Yb3+ codoped La2O3 phosphor was investigated. Decay curve analysis of the prepared phosphors was done to understand the mechanism of the upconversion emission intensity variation due to codoping with Zn2+/Mg2+ ions. The purity of colour emitted from the sample does not show any change with variations in pump power density.

 Please wait while we load your content...
Please wait while we load your content...