Gas phase hydration of amino acids and dipeptides: effects on the relative stability of zwitterion vs. canonical conformers
Abstract
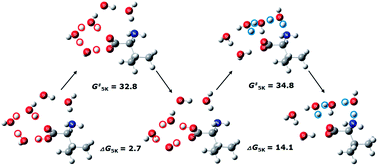
We present a brief review of studies of the relative stability of canonical vs. zwitterionic forms of amino acids and dipeptides under the influence of gas phase hydration. Focus is given on the number of water molecules necessary to stabilize the zwitterionic conformer. Experimental and theoretical investigations for this interesting question are discussed. It is shown that the hydrating properties of amino acids and dipeptides are strongly dependent on the characteristics (hydrophilicity, basicity etc.) of side chains, the presence of metal cations, or an excess electron. Besides the relative Gibbs free energies of various conformers to estimate their relative thermodynamic stability, the activation barriers of proton transfer processes between canonical and zwitterionic forms are emphasized to assess the kinetic stability of thermodynamically less favorable species in low-temperature, gas phase environments.

 Please wait while we load your content...
Please wait while we load your content...