Controlled synthesis of hierarchical MnO2 microspheres with hollow interiors for the removal of benzene†
Abstract
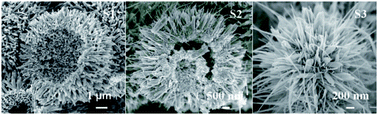
Three types of MnO2 microspheres, viz., hierarchical hollow β-MnO2 microspheres consisting of uniform nanorods, hierarchical double-walled hollow β/α-MnO2 microspheres assembled by two-categorical nanorods, and hierarchical hollow α-MnO2 microspheres constructed by nanorods and nanowires, have been synthesized by a facile hydrothermal method without employing any templates, catalysts, surfactants or calcinations. A number of characterization techniques, including X-ray diffraction (XRD), field-emission scanning electron microscopy (FESEM), energy dispersive spectroscopy (EDS), transmission electron microscopy (TEM), nitrogen adsorption–desorption measurements, and X-ray photoelectron spectroscopy (XPS) are used to characterize the formed hierarchical products, and to address the following critical issues: (i) effect of the precursor concentration; (ii) the role of HCl for the formation of the hierarchical hollow structure; and (iii) the possible mechanism accounting for the formation of the hierarchical hollow microspheres. The hierarchical hollow MnO2 microspheres can be used as catalysts for the catalytic oxidation of benzene, of which the hierarchical hollow α-MnO2 microspheres exhibit the highest catalytic ability.

 Please wait while we load your content...
Please wait while we load your content...