Efficient catalytic-free method to produce α-aryl cycloalkanones through highly chemoselective coupling of aryl compounds with oxyallyl cations†
Abstract
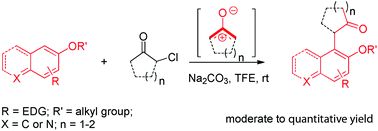
Catalytic-free coupling of aryl compounds and α-halo cycloketones via in situ generated oxyallyl cation intermediates is reported here. The reactions efficiently afford α-naphthol cycloalkanones with moderate to excellent yields. Electron-rich aromatic compounds are also used to produce the corresponding α-aryl cycloalkanones, and in some cases, analytically pure products are obtained after simple filtration followed by evaporation.

 Please wait while we load your content...
Please wait while we load your content...