Pyrolysis of azetidinone derivatives: a versatile route towards electron-rich alkenes, C-1 allylation and/or homologation of aldehydes†
Abstract
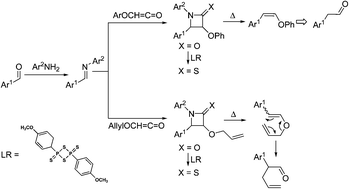
Pyrolysis of β-lactams and β-thiolactams led essentially to stereoselective synthesis of the high energy electron-rich Z-alkenes. Extension of this methodology to the pyrolysis of 3-allyloxy derivatives gave a simple direct route to the synthetically important 4-pentenal. These pyrolytic transformations convert aldehydes to aryloxyalkenes (a protected homologation) and 4-pentenal (a C-1 allylation and homologation). The starting 3-aryloxy and 3-allyloxy-β-lactams were synthesized by the standard Staudinger ketene-imine [2 + 2] cycloaddition. The corresponding β-thiolactams have readily been obtained in good yields by thiation of β-lactams with Lawesson's reagent.

 Please wait while we load your content...
Please wait while we load your content...