A protecting group free and scalable approach towards total synthesis of (−)-venlafaxine†
Abstract
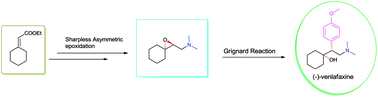
A protecting group free asymmetric total synthesis of (−)-venlafaxine is reported. The strategy employs Sharpless epoxidation and regio-selective epoxide ring opening by an in situ generated Gilman reagent as key steps. This paper reports a 53% overall yield in 6 steps for total synthesis of (−)-venlafaxine.

 Please wait while we load your content...
Please wait while we load your content...