Rapid production of silver nanowires based on high concentration of AgNO3 precursor and use of FeCl3 as reaction promoter
Abstract
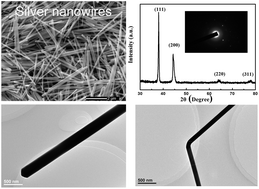
Massive silver nanowires with high yields have been synthesized within 10 minutes by a facile and effective two-step dropwise addition polyol method due to high reaction rates by virtue of high AgNO3 concentrations and anti-oxidation properties of the reaction promoter FeCl3. In particular, pure nanowires could be obtained by varying the AgNO3 concentrations from 0.1 M to 0.6 M. It was found that the concentrations of Fe3+, Cl−, and PVP, bubbling atmosphere, reaction temperature, and time were important factors for controlling the morphology of the products. The results showed that a FeCl3 concentration of 0.1–0.15 mM, a PVP concentration of 0.3–0.45 M, air bubbling atmosphere, a temperature of 150–160 °C, and a reaction time of 10–120 min were appropriate conditions for the synthesis of nanowires, where the diameters could be tuned from 80 nm to 323 nm, and the length could be tuned from 3.7 μm to 14.3 μm. Moreover, Fe3+ could accelerate and facilitate the formation of silver nanowires by preventing their oxidative etching, while excessive Fe3+ also etched silver nanostructures. Furthermore, the rapid growth of nanowires due to a sufficient silver source provided by the second rapid dropwise addition and etching of other particles by Cl−/O2 occurred simultaneously under air, thereby producing high-quality silver nanowires. Additionally, the diameter of the silver nanowires decreased and their surfaces became rougher, which is ascribed to etching effects with the time. More specifically, two silver nanowires could fuse together to form long nanowires arising from the existence of high nitrate anion concentrations. The size-dependent UV-VIS absorption spectra displayed that the surface plasmon resonance (SPR) peak broadened and red shifted from 384 nm to 458 nm with an increase in diameter from 80 nm to 323 nm. Meanwhile, the number of SPR peaks decreased from two to one. In consideration of morphology controllability, less time and energy cost, and synthesis of massive nanowires, the method is promising for realizing the industrial production of silver nanowires.

 Please wait while we load your content...
Please wait while we load your content...