One-flask synthesis of 1,3,5-trisubstituted 1,2,4-triazoles from nitriles and hydrazonoyl chlorides via 1,3-dipolar cycloaddition†
Abstract
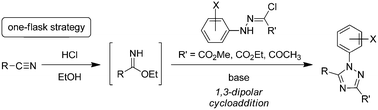
A one-flask strategy for the synthesis of 1,3,5-trisubstituted 1,2,4-triazoles 4a–s and 8a and b from nitriles 5a–i with N-arylhydrazonoyl hydrochlorides 3a–h and 7a and b under basic conditions was developed. The reaction provided the desired 1,2,4-triazoles in moderate to excellent yields (56–98%), and was applicable to aliphatic and aromatic nitriles as well as N-phenylhydrazonoyl hydrochlorides bearing ester and acetyl functionalities. A 1,3-dipolar cycloaddition between imidate and nitrilimine generated from the respective nitrile and N-arylhydrazonoyl chloride in one flask was proposed for the new transformation.

 Please wait while we load your content...
Please wait while we load your content...