Palladium(ii) induced complete conformational enrichment of the syn isomer of N,N′-bis(4-pyridylformyl)piperazine†
Abstract
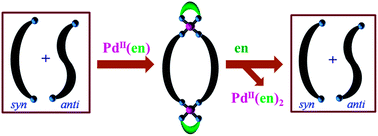
In the solution state, an equimolar mixture of syn- and anti-conformations is present for the ligand N,N′-bis(4-pyridylformyl)piperazine, L. Combining L with cis-Pd(en)(NO3)2 resulted in the formation of metallomacrocycle cis-[Pd2(en)2(L)2](NO3)4, 1, quantitatively. Coordinated L in complex 1 was found to exist exclusively in the syn-conformation. The equimolar mixture of syn- and anti-conformations was re-established by ethylenediamine induced demetallation of 1.

 Please wait while we load your content...
Please wait while we load your content...