Self-assembly and phase separation of amphiphilic dyads based on 4,7-bis(2-thienyl)benzothiodiazole and perylene diimide†
Abstract
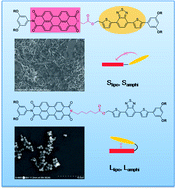
To get highly ordered organic structures at the nanoscale, a series of new electronic donor–acceptor (D–A) dyads were synthesized. The dyads bearing different side chains (lipophilic or amphiphilic) or linkers (Long or Short) showed variable self-assembly behaviour. Long-linker dyads can fold in dilute solution, but the folding of short-linker dyads was not observed. Intermolecular D–A interactions and acceptor–acceptor (A–A) aggregation were proved to co-occur in chloroform for all four dyads. In the bulk state, amphiphilic dyads could overcome the intrinsic D–A interactions and achieve better D–A phase separation than lipophilic ones due to the incompatibility of the side chains. The dyad with a short-linker and amphiphilic side chains, Samphi, had the ability to form a gel, and both of the amphiphilic dyads could form nanofibres.

 Please wait while we load your content...
Please wait while we load your content...