Oxazoline derivatives tagged with tosylated amino acids as recyclable organocatalysts for enantioselective allylation of aldehydes†
Abstract
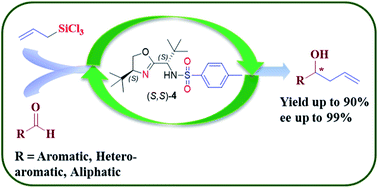
A series of amino acid-based oxazoline compounds have been prepared and successfully applied to the enantioselective allylation reaction of aldehydes. The fine-tuning of the structure of the oxazolines led to (S,S)-4 as an efficient organocatalyst which gave homoallyl alcohols in good yield (up to 90%) and excellent ee (up to 99%) for a wide range of substrates including aromatic, hetero-aromatic and α,β-unsaturated aldehydes. The chiral organocatalyst was synthesized in three easy steps with an overall 88% yield and successfully recycled for up to three cycles. On the basis of the experimental observations and NMR studies, a probable mechanism was proposed for this reaction.

 Please wait while we load your content...
Please wait while we load your content...