Three-step assembly of 4-aminotetrahydropyran-2-ones from isoxazoline-2-oxides†
Abstract
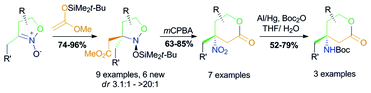
Tetrahydropyran-2-ones with a 4-amino function connected to a tertiary carbon atom – a widely naturally occurring fragment – are constructed by a three step protocol from easily available isoxazoline 2-oxides. In the first stage, the carbon skeleton of the target product is formed upon a C,C-coupling of a silyl ketene acetal with a nitronate function, under silyl triflate catalysis. The key step of the assembly consists of the oxidative cleavage of an endocyclic N–O bond of intermediate cyclic nitroso acetals with mCPBA, accompanied with lactone ring closure, and gives rise to β-nitro-δ-lactones in 63–85% yields. The latter are reduced with amalgamated aluminium, to furnish the target scaffold.

 Please wait while we load your content...
Please wait while we load your content...