Five copper(ii) metal–organic coordination complexes with micro-channels based on flexible bis(imidazole) and carboxybenzaldehyde ligands; structural influence of experimental conditions on their frameworks†
Abstract
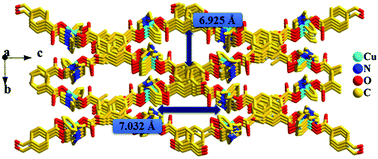
Five new copper(II) metal–organic coordination complexes, [Cu2(1,2-bix)2(L)4] (1), [Cu(1,3-bix)(L)2]n·nCH3OH·3H2O (2), [Cu(1,3-bix)(L)2]n·nH2O (3), [Cu5(1,3-bix)2(L)6(NO3)2(H2O)2(OH)2]·2H2O (4) and [Cu(1,4-bix)(L)2]n (5), have been synthesized using 4-carboxybenzaldehyde (HL) and 1,n-bis(imidazol-l-ylmethyl)benzene (1,n-bix; n = 2, 3, 4) as ligands. These complexes were structurally characterized by single-crystal X-ray crystallography and further characterized by FT-IR spectroscopy and thermogravimetric analysis (TGA). Complex 1 is a symmetrical binuclear structure, and in compound 2 the 1D ladders are entangled through π–π stacking interactions to generate a 3D network with one-dimensional micro channel. A 3D coordination framework 2 converted to network 3 by just increasing the reaction temperature. Metal–organic polymer 3 is comprised of 1D corrugated double chains structure. Complex 4 has a pentanuclear (Cu5) cluster structure and compound 5 shows a 1D zigzag chain. The magnetic properties of the complexes 1 and 3 are reported briefly.

 Please wait while we load your content...
Please wait while we load your content...