A non-singlet oxygen mediated reaction photoinduced by phenalenone, a universal reference for singlet oxygen sensitization†
Abstract
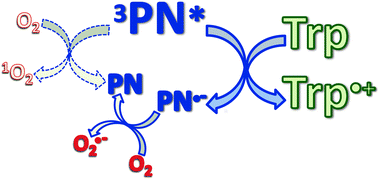
Photosensitized oxidations involve the generation of radicals (type I mechanism) and/or the production of singlet molecular oxygen (1O2) (type II mechanism). Phenalenone (PN) is one of the well-known type II photosensitizers and is widely used as a 1O2 reference sensitizer. In this work, we have studied the interaction of tryptophan (Trp) with the excited states of PN, the production of 1O2 in the presence of Trp, the formation of radicals and the kinetics of the photodegradation of Trp under different experimental conditions. In contrast to what is usually assumed, we have proven that PN is also able to act as a type I photosensitizer. Moreover, we have demonstrated that the predominant mechanism of the photosensitization of Trp by PN involves an electron transfer initiated-process.

 Please wait while we load your content...
Please wait while we load your content...