Copper-promoted cascade reaction of active methylenes with MBH-acetates of acetylenic aldehydes to functionalized cyclopentenes†
Abstract
One-pot synthesis of substituted cyclopentenes was accomplished through a copper-mediated [4 + 1]-annulation of active methylene compounds with Morita–Baylis–Hillman (MBH) acetates of acetylenic aldehydes. The reaction sequence involves the cascade allylic substitution/5-exo-dig-carbocyclization, which was effective in providing methylene, alkylidene as well as arylidene cyclopentenes.
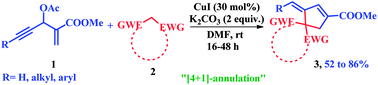

 Please wait while we load your content...
Please wait while we load your content...