Enantioselective oxidation of secondary alcohols by Candida parapsilosis ATCC 7330
Abstract
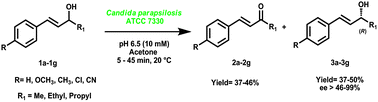
Optically pure allylic alcohols and 4-phenylbutan-2-ols were prepared by oxidative kinetic resolution using whole cells of Candida parapsilosis ATCC 7330. Only the ‘S’ enantiomer is selectively oxidized to the corresponding keto compound (yield, 37–46%) leaving the ‘R’ alcohol (yield, 37–52% and ee 46 to >99%). The biocatalytic method is carried out under mild conditions at 20 °C, pH 6.5 in phosphate buffer.

 Please wait while we load your content...
Please wait while we load your content...