A fluoride ion selective Zr(iv)-poly(acrylamide) magnetic composite†
Abstract
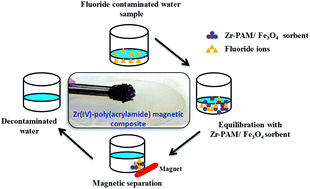
A fluoride ion selective magnetic sorbent has been synthesized by the encapsulation of Fe3O4 nanoparticles in a network of Zr(IV) complexed poly(acrylamide) (Zr–PAM). This magnetic sorbent has been found to be efficient for the selective preconcentration of fluoride ions from natural waters. The Zr–PAM/Fe3O4 composite has been characterized using various physico-chemical techniques i.e. energy dispersive X-ray fluorescence (EDXRF), scanning electron microscopy (SEM), Fourier transform infra-red spectroscopy (FTIR) and a vibrating sample magnetometer (VSM). The Zr–PAM/Fe3O4 composite developed in the present work retains the super paramagnetic properties of Fe3O4 nanoparticles, and the results reveal that the sorption is rapid. The composite has a considerably higher fluoride sorption capacity (124.5 mg g−1) compared to other super-paramagnetic fluoride sorbents reported in the literature. Repeated sorption–regeneration cycles seem to suggest reusability of the sorbent for fluoride removal from natural waters, as well as other aqueous solutions having pH in the range 1–9.

 Please wait while we load your content...
Please wait while we load your content...