Unveiling the dual role of the cholinium hexanoate ionic liquid as solvent and catalyst in suberin depolymerisation†
Abstract
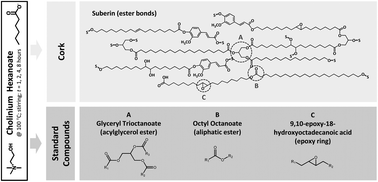
Disruption of the three-dimensional network of suberin in cork by cholinium hexanoate leads to its efficient and selective isolation. The reaction mechanism, which likely involves selective cleavage of some inter-monomeric bonds in suberin, was still unanswered. To address this question, the role of the ionic liquid during suberin depolymerisation and during cleavage of standard compounds carrying key chemical functionalities was herein investigated. A clear demonstration that the ionic liquid catalyses the hydrolysis of acylglycerol ester bonds was attained herein, both experimentally and computationally (DFT calculations). This behaviour is related to cholinium hexanoate capacity to activate the nucleophilic attack of water. The data showed also that the most favourable reaction is the hydrolysis of acylglycerol ester bonds, with the C2 position reporting the faster kinetics, whilst most of the linear aliphatic esters remained intact. The study emphasises that the ionic liquid plays the dual role of solvent and catalyst and leads to suberin efficient extraction through a mild depolymerisation. It is also one of the few reports of ionic liquids as efficient catalysts in the hydrolysis of esters.

 Please wait while we load your content...
Please wait while we load your content...