Destabilization of LiBH4 dehydrogenation through H+–H− interactions by cooperating with alkali metal hydroxides†
Abstract
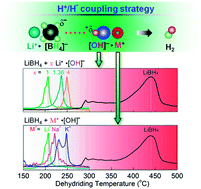
Destabilization by the alkali metal hydroxides LiOH, NaOH, and KOH in the solid-state dehydrogenation of LiBH4 is reported. 6.5 wt% of hydrogen was liberated within 10 minutes at 250 °C. Destabilization originated from the interaction between H+ in [OH]− and H− in [BH4]−. A larger Pauling's electronegativity of the alkali metal (Li > Na > K) led to a greater acidity of the proton donor [OH]− site, and thus enhanced destabilization. The temperature of the predominant dehydrogenation was reduced to 207, 221, and 230 °C, for ball milled LiBH4–LiOH, 2LiBH4–NaOH, and 2LiBH4–KOH, respectively. The LiBH4: LiOH stoichiometry greatly affected the destabilization, by providing differing reaction pathways in LiBH4-xLiOH (x = 1, 1.36, 4). The incremental increase in the LiOH content of LiBH4-xLiOH increased the dehydrogenation rate, but the temperature increased from 207 °C (x = 1) to 250 °C (x = 4). 4.1 and 6.5 wt% of hydrogen was liberated within 10 minutes by LiBH4–LiOH and LiBH4–4LiOH, respectively. The incremental increase in dehydrogenation temperature was attributed to differing [BH4]−⋯[OH]− interactions, formed by the differing stoichiometric ratios.

 Please wait while we load your content...
Please wait while we load your content...