Epoxy amino acids produced from allylglycines intramolecularly cyclised to yield four stereoisomers of 4-hydroxyproline derivatives†
Abstract
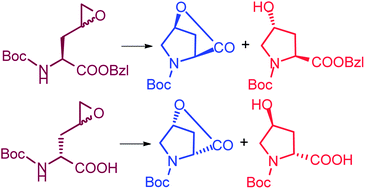
Derivatives of 2-amino-4-pentenoic acid (allylglycine) were efficiently resolved using Subtilisin or acylase. The side-chain unsaturated bond of the enantiomerically pure amino acid with tert-butoxycarbonyl (Boc) protection was smoothly epoxidized with m-chloroperbenzoic acid. When the Boc protection of the amino group was removed, the amino group intramolecularly attacked the side-chain epoxide, generating compounds with five-membered rings: the 4-hydroxyproline derivatives. Two diastereomeric products were formed through the cyclisation reaction, for example, (2S,4S)-4-hydroxyproline benzyl ester (cis-8) and (2S,4R)-4-hydroxyproline benzyl ester (trans-8) were formed from (2S)-amino acid with a side-chain epoxide. Compound (2S,4S)-4-hydroxyproline benzyl ester (cis-8) was transformed to a lactone (cis-hydroxyproline lactone, 10) with the removal of benzyl alcohol. The cis-conformation was essential for the intramolecular ester exchange reaction; in fact, no lactone formation was observed for the trans isomer (trans-8). The separation of cis-hydroxyproline lactone and the trans-isomeric hydroxyproline benzyl ester was facile and clear, in contrast to the difficult separation of cis- and trans-hydroxyproline derivatives. Thus, two diastereomers of hydroxyproline derivatives for L-hydroxyproline and also for D-hydroxyproline were obtained, i.e., four diastereomers of hydroxyproline derivatives.

 Please wait while we load your content...
Please wait while we load your content...