Ultrasound-promoted synthesis of bi-, tri- and tetrapodal polyhydroquinolines, 1,4-dihydropyridines and the corresponding pyridines†
Abstract
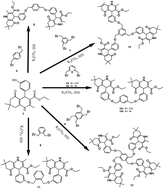
Bi-, tri- and tetrapodal polyhydroquinolines and 1,4-dihydropyridines were synthesised by the reaction of various alkylating agents with polyhydroquinolines and 1,4-dihydropyridines under sonication conditions. These were in turn converted to the corresponding pyridines also using sonication. Sonication produced higher yields in a faster reaction time. All the synthesized compounds were characterized using spectral data.

 Please wait while we load your content...
Please wait while we load your content...