Enhancement of anti-leukemic potential of 2-hydroxyphenyl-azo-2′-naphthol (HPAN) on MOLT-4 cells through conjugation with Cu(ii)†
Abstract
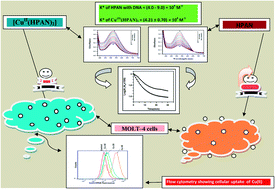
A Cu(II) complex of 2-hydroxyphenyl-azo-2′-naphthol (HPAN) having the formula CuII(HPAN)2 was characterized by different techniques. When HPAN and CuII(HPAN)2 were incubated for 24 hours with human T-acute lymphoblastic leukemia (MOLT-4) cells, almost no activity was observed for HPAN while the complex was active. When incubated for 48 hours, HPAN showed cell death of ∼35% at a concentration of 40 μM while CuII(HPAN)2 was only slightly better than when incubated for 24 hours. Therefore, irrespective of incubation time, the anti-proliferative activity due to CuII(HPAN)2 was similar. However, increase in incubation time did show increased activity for HPAN. Anti-leukemic potential was confirmed by microscopic analysis of cell viability by trypan blue stain and MTT assay. The BrdU assay further confirmed proliferative effects of aqueous Cu(II)/HPAN and anti-proliferative effects of Cu(II)(HPAN)2. Propidium iodide staining of Cu(II)(HPAN)2-treated MOLT-4 cells confirmed apoptosis. Since amines formed as a consequence of reduction of the azo bond are reported to be cytotoxic, we performed an enzyme assay to understand the relative reduction of the azo bond in both compounds. Results suggest reduction of the azo bond was slightly higher for HPAN. DNA binding of CuII(HPAN)2 using fluorescence spectroscopy was compared with that of HPAN to determine the propensity of biological activity. The results being similar, binding of the compounds with DNA and the ease of reduction of the azo bond were not able to explain why CuII(HPAN)2 was better in preventing cell proliferation. The high anti-proliferative activity of CuII(HPAN)2 was attributed to increased cellular uptake. We designed experiments to support this hypothesis using independent approaches. In one, Cu(II) was identified in cell lysates using ferrocyanide, while, in another, CuII(HPAN)2 was detected using flow cytometry. We chose Cu(II) as the metal ion for this work because of its recognized involvement in cancer. Being essential for angiogenesis, it is found in increased levels in cancer cells. Interaction of Cu(II)(aq) with MOLT-4 cells confirmed this as a part of this study also. Hence, our objective was to see if molecules like HPAN that bind Cu(II) could lead to its role reversal, i.e. from supporting the growth of cancer cells to be able to destroy them as CuII(HPAN)2.

 Please wait while we load your content...
Please wait while we load your content...