Unprecedented single-pot protocol for the synthesis of novel bis-3,4-dihydropyrimidin-2(1H)-ones using PEG-HClO4 as a biodegradable, highly robust and reusable solid acid green catalyst under solvent-free conditions†
Abstract
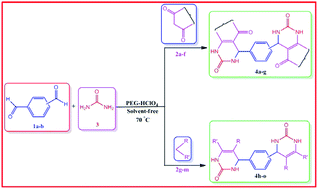
A solvent-free, environmentally benign, and simple one-pot multi-component protocol has been developed for the efficient synthesis of novel bis-3,4-dihydropyrimidin-2(1H)-one derivatives (4a–o) in excellent yields using perchloric acid-modified PEG-6000 (PEG-HClO4) as a biodegradable and reusable catalyst at ambient temperature. This novel and green protocol has the advantages of shorter reaction times, high conversions, use of an inexpensive catalyst and simple experimental procedure, as compared to the conventional methods. The PEG-HClO4 catalyst is characterized by using FTIR, powder XRD and SEM-EDX analyses. The catalyst can be reused several times without significant loss of its catalytic activity.

 Please wait while we load your content...
Please wait while we load your content...