Unusual chemical transformations of acetone thiosemicarbazone mediated by ruthenium: C–H bond activation, thiolation, and C–N bond cleavage†
Abstract
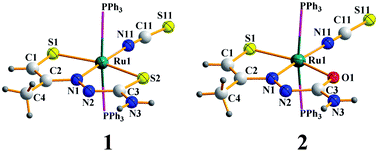
Upon reaction with Ru(PPh3)3Cl2 in ethanol in the presence of triethylamine, acetone thiosemicarbazone undergoes several interesting chemical transformations, such as thiolation via methyl C–H bond activation, C–N bond cleavage, and conversion of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S fragment to C
S fragment to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O. Two complexes (1 and 2) were obtained from this reaction, both of which contained a modified thiosemicarbazone coordinated in SNS- or SNO-mode, two triphenylphosphines and a N-bound thiocyanate. The crystal structures of both the complexes have been determined. Theoretical and mass spectral studies have been carried out to probe the transformations. These complexes show intense absorptions in the visible and ultraviolet regions. Cyclic voltammetry on both the complexes show a reversible oxidation near 0.6 V vs. SCE, followed by an irreversible oxidation near 1.2 V vs. SCE. DFT calculations have been carried out to explain the electronic spectra, as well as the electrochemical observations.
O. Two complexes (1 and 2) were obtained from this reaction, both of which contained a modified thiosemicarbazone coordinated in SNS- or SNO-mode, two triphenylphosphines and a N-bound thiocyanate. The crystal structures of both the complexes have been determined. Theoretical and mass spectral studies have been carried out to probe the transformations. These complexes show intense absorptions in the visible and ultraviolet regions. Cyclic voltammetry on both the complexes show a reversible oxidation near 0.6 V vs. SCE, followed by an irreversible oxidation near 1.2 V vs. SCE. DFT calculations have been carried out to explain the electronic spectra, as well as the electrochemical observations.

 Please wait while we load your content...
Please wait while we load your content...