The exploration of Kemp's triacid (KTA) as the core for the synthesis of 3-fold symmetric 23-cyclophane, 22-cyclophane and novel linker directed designs†
Abstract
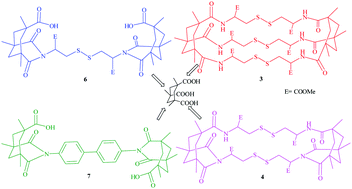
For the first time, two units of KTA have been linked to three units of cyst-di-OMe. The reaction is noteworthy since it involves the formation of six amide bonds leading to a three-fold symmetric 23-cyclophane (3) harboring a cluster of three S–S bridges. The major product is a di-imide (4), arising from the interaction of a cystine NH with a neighbouring activated ester. A third reaction of tethering KTA with a single cyst-di-OMe unit afforded the flexible compound 6 and, with benzidine, the novel linker directed 7 with orthogonally disposed anchor modules.

 Please wait while we load your content...
Please wait while we load your content...