Syntheses, biological evaluation and photophysical studies of novel 1,2,3-triazole linked azo dyes†
Abstract
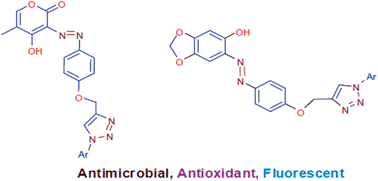
We have reported synthesis of two novel series of 1,2,3-triazole linked azo dyes using 4-hydroxy-5-methyl-2H-pyran-2-one and sesamol as coupling components in view of the increasing resistance to existing antimicrobial drugs and the need for new antioxidant agents and fluorescent materials. All newly synthesized compounds were evaluated for antibacterial activity, antifungal activity, antioxidant activity and photophysical properties. Antimicrobial activity was evaluated against six microbial strains. Compound 6a was found to be the most potent antibacterial and antifungal agent, while other compounds showed good to moderate antimicrobial activity. Antioxidant activity was evaluated using DPPH free radical scavenging assay and nitric oxide radical scavenging assay. Compound 6b was found to be 5 fold more potent than standard antioxidant BHT. Other compounds showed good antioxidant activity. Photophysical properties of all compounds were also investigated in detail.

 Please wait while we load your content...
Please wait while we load your content...