Synthesis of triazole linked fluorescent amino acid and carbohydrate bio-conjugates: a highly sensitive and skeleton selective multi-responsive chemosensor for Cu(ii) and Pb(ii)/Hg(ii) ions†
Abstract
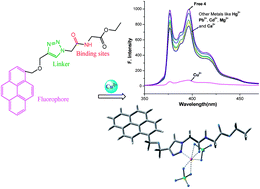
A set of triazole-based chromogenic and fluorescent chemosensors with amino acid/carbohydrate–fluorophore conjugates have been designed and synthesized. The metal cation-sensing properties of glycine–anthracene, C24H24O4N4 (3), glycine–pyrene C26H24O4N4 (4), glucose–anthracene, C32H33O10N3 (5) and glucose–pyrene, C34H33O10N3 (6) bio-conjugates have been studied systematically. The significant changes in their absorption spectra are accompanied by a strong color change from light yellow to brown for 3 and 4 and colorless to greenish blue for 5 and 6. Receptors 3 and 4 have potential in the “naked eye” detection of Cu2+ and 5 and 6 for Pb2+/Hg2+ ion. The receptors 3 and 4 show fluorescence diminution following Cu2+ coordination within the limit of detection at 0.89 parts per billion (ppb) and this is unprecedented, whereas the receptors 5 and 6 present drastic fluorescence quenching upon addition of Hg2+ and Pb2+ within the limit of detection at 4 and 2 ppb respectively. Interestingly, their fluorescence and colorimetric responses are preserved in the presence of water that can be used for the selective colorimetric detection of these ions in aqueous environments. Along with the spectroscopic data, combined 1H NMR titration of the complexes and the DFT studies suggest the proposed coordination modes.

 Please wait while we load your content...
Please wait while we load your content...