A problem solving approach for the diastereoselective synthesis of (5′S)- and (5′R)-5′,8-cyclopurine lesions†‡
Abstract
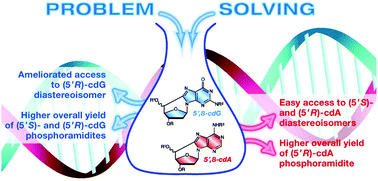
A short, efficient and high-yielding approach for the diastereoselective syntheses of the four (5′S)- and (5′R)-5′,8-cyclopurine lesions and their phosphoramidites has been developed. The intermediates prepared by these synthetic pathways provide an easy access to those modified nucleosides that constitute an important molecular library for recognition of DNA damage. With respect to previous synthesis, the key methodologies for cyclization steps and phosphoramidite synthesis were ameliorated.
- This article is part of the themed collections: HOT articles in Organic Chemistry Frontiers for 2014 and In Celebration of Max Malacria’s 65th Birthday

 Please wait while we load your content...
Please wait while we load your content...