Palladium-catalyzed amination of allylic carbonates with ammonia: access to primary amines†
Abstract
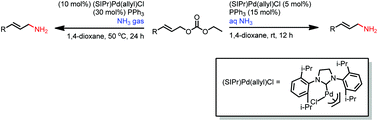
Allylic amination of allylic carbonates with ammonia gas or aqueous ammonia was successfully carried out using (SIPr)Pd(allyl)Cl as the catalyst and Ph3P, which is essential for the reaction. The aqueous ammonia reactions proceeded smoothly at room temperature, using a low catalyst loading. But a higher temperature and catalyst loading were needed for the corresponding ammonia gas reactions. Moderate to good yields were achieved for both reactions. This study demonstrates the feasibility of using ammonia as an aminating reagent, and it opens the field for further development of metal-catalyzed allylic amination in the future.

 Please wait while we load your content...
Please wait while we load your content...