Ferrocene as a functional group enhances the inhibitive effect of dihydropyrimidine on radical-induced oxidation of DNA
Abstract
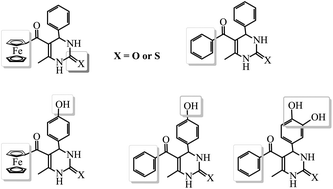
Phenyl and ferrocenyl groups are involved in 3,4-dihydropyrimidin-2(1H)-one (-thione) to form eleven dihydropyrimidines (DHPMs) in this work, aiming to explore the effects of the ferrocene moiety, the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S bond, and the phenolic hydroxyl group on the abilities of DHPMs to protect DNA against 2,2′-azobis(2-amidinopropane hydrochloride) (AAPH)-induced oxidation. It is found that the antioxidant abilities of DHPMs containing C
S bond, and the phenolic hydroxyl group on the abilities of DHPMs to protect DNA against 2,2′-azobis(2-amidinopropane hydrochloride) (AAPH)-induced oxidation. It is found that the antioxidant abilities of DHPMs containing C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S are higher than those of DHPMs containing C
S are higher than those of DHPMs containing C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O. In addition, the phenolic hydroxyl group and the C
O. In addition, the phenolic hydroxyl group and the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S bond show similar effects on the antioxidant capacities of DHPMs. On the other hand, the ferrocenyl group remarkably increases the antioxidant capacities of DHPMs, while the antioxidant effects of ferrocenyl-appended DHPMs are further improved by the C
S bond show similar effects on the antioxidant capacities of DHPMs. On the other hand, the ferrocenyl group remarkably increases the antioxidant capacities of DHPMs, while the antioxidant effects of ferrocenyl-appended DHPMs are further improved by the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S bond. Therefore, the influence of the functional group on the antioxidant abilities of DHPMs follows the order: ferrocenyl group > C
S bond. Therefore, the influence of the functional group on the antioxidant abilities of DHPMs follows the order: ferrocenyl group > C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S bond > phenolic hydroxyl group.
S bond > phenolic hydroxyl group.

 Please wait while we load your content...
Please wait while we load your content...