Highly enantioselective oxidative tandem cyclization reaction: a chiral ligand and an anion cooperatively control stereoselectivity†
Abstract
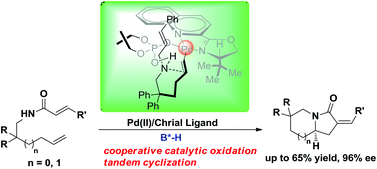
The unprecedented combination of a palladium(II) complex with a chiral Bu-QUOX ligand and a chiral phosphoric acid enables the highly efficient asymmetric oxidative tandem cyclization of N-(2,2-disubstituted hex-5-en-1-yl)acrylamides, providing a straightforward method to access chiral 6,5-bicyclic aza-heterocycles in moderate to good yields and with excellent enantioselectivities.

 Please wait while we load your content...
Please wait while we load your content...