Hydrogen bonding-driven highly stable homoduplexes formed by benzene/naphthalene amide oligomers†
Abstract
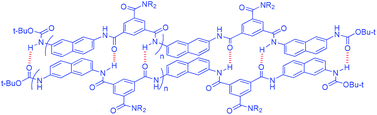
This paper describes the dimerization of aromatic amide oligomers in chloroform. The oligomers were prepared by step-by-step coupling of benzene-m-dicarboxylic acid and naphthalene-2,7-diamine. Triethylene glycol chains were introduced into the 5-position of the benzene rings to provide solubility in organic solvents. 1H NMR investigations revealed that, although shorter oligomers did not exhibit good dimerization ability, longer oligomers with five or more amide units could form stable homoduplexes which were stabilized by intermolecular multiple N–H⋯O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C hydrogen bonds. The association constants (Ka) of the 3-, 4- and 5-mer oligomers were determined using 1H NMR dilution experiments, while the Ka values of a 5-mer oligomer and a 7-mer oligomer, which were appended with two or one pyrene unit, were also evaluated using fluorescent dilution experiments. The results showed that the stability of the homoduplexes of the aromatic amide oligomers is nearly linearly related to the number of their amide units, and a Ka of 3.0 × 107 M−1 was determined for the 7-mer oligomer.
C hydrogen bonds. The association constants (Ka) of the 3-, 4- and 5-mer oligomers were determined using 1H NMR dilution experiments, while the Ka values of a 5-mer oligomer and a 7-mer oligomer, which were appended with two or one pyrene unit, were also evaluated using fluorescent dilution experiments. The results showed that the stability of the homoduplexes of the aromatic amide oligomers is nearly linearly related to the number of their amide units, and a Ka of 3.0 × 107 M−1 was determined for the 7-mer oligomer.

 Please wait while we load your content...
Please wait while we load your content...