Palladium-catalyzed base-accelerated direct C–H bond alkenylation of phenols to synthesize coumarin derivatives†
Abstract
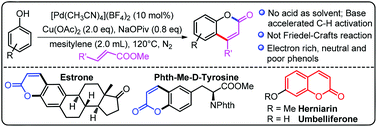
A palladium-catalyzed base-accelerated ortho-selective C–H alkenylation of phenols to synthesize bioactive coumarin derivatives was developed. The reaction condition was mild and the substrate scope was broad with both electron-neutral and electron-deficient phenols, which is complementary to the previous methods to synthesize electron-rich coumarins. Several bioactive molecules were functionalized and several coumarins with bioactive properties were synthesized. Mechanistic studies showed that this reaction underwent C–H bond activation via direct metallation rather than the Friedel–Crafts pathway.
- This article is part of the themed collection: HOT articles in Organic Chemistry Frontiers for 2014

 Please wait while we load your content...
Please wait while we load your content...