Catalytic Sonogashira couplings mediated by an amido pincer complex of palladium†
Abstract
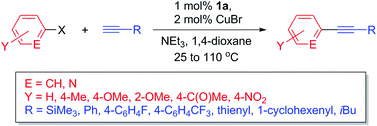
This work describes the efficacy of [PNP]PdCl (1a), where [PNP]− = bis(2-diphenylphosphinophenyl)amide, as a catalyst precursor for Csp–Csp2 bond-forming cross-coupling reactions of terminal alkynes with aryl halides in the presence of copper bromide and aliphatic amines in ethereal solutions under mild conditions. This catalysis is compatible with acetylenes that are alkyl, alkenyl, (hetero)aryl, or silyl substituted and aryl iodides or bromides that are electronically activated, neutral, or deactivated. The low reaction constants of 0.82(6) and 0.97(7) obtained from Hammett plots of competitive reactions employing electronically distinct aryl iodides and terminal alkynes, respectively, are likely suggestive of irrelevance of the rate-determining step in these catalytic transformations to oxidative addition of aryl halides or generation of mono-substituted acetylides. In sharp contrast, reactions employing a phosphorus-bound isopropyl derived 1b gave rather unsatisfactory results, highlighting a profound phosphorus substituent effect on this aryl alkynylation catalysis.

 Please wait while we load your content...
Please wait while we load your content...