Interaction of alkynes with palladium POCOP-pincer hydride complexes and its unexpected relation to palladium-catalyzed hydrogenation of alkynes†
Abstract
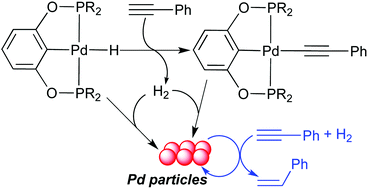
Palladium POCOP-pincer hydride complexes [2,6-(R2PO)2C6H3]PdH (R = tBu, 2a; R = iPr, 2b; R = cPe, 2c, cPe = cyclopentyl) have been synthesized from [2,6-(R2PO)2C6H3]PdCl (1a–c) and LiAlH4 or LiBEt3H. These hydride complexes react with phenylacetylene to afford H2, [2,6-(R2PO)2C6H3]PdC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CPh (3a–c) and a small amount of styrene. When the R groups are isopropyl groups, a second palladium species is generated, and it has been identified as an alkenyl complex (E)-[2,6-(iPr2PO)2C6H3]PdCH
CPh (3a–c) and a small amount of styrene. When the R groups are isopropyl groups, a second palladium species is generated, and it has been identified as an alkenyl complex (E)-[2,6-(iPr2PO)2C6H3]PdCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHPh (4b). Mechanistic studies have shown that decomposition of these palladium pincer complexes and related palladium methyl complexes [2,6-(R2PO)2C6H3]PdCH3 (5a–c) occurs at room temperature in the presence of H2 (1 atm or lower), resulting in the leaching of palladium particles. These particles have been shown to catalyze the hydrogenation of phenylacetylene and diphenylacetylene to their alkene and alkane products. A mechanism for the formation of palladium particles has been proposed. The structures of 1a, 1c, 2a, 2c, 3a, 4b and 5b have been studied by X-ray crystallography.
CHPh (4b). Mechanistic studies have shown that decomposition of these palladium pincer complexes and related palladium methyl complexes [2,6-(R2PO)2C6H3]PdCH3 (5a–c) occurs at room temperature in the presence of H2 (1 atm or lower), resulting in the leaching of palladium particles. These particles have been shown to catalyze the hydrogenation of phenylacetylene and diphenylacetylene to their alkene and alkane products. A mechanism for the formation of palladium particles has been proposed. The structures of 1a, 1c, 2a, 2c, 3a, 4b and 5b have been studied by X-ray crystallography.

 Please wait while we load your content...
Please wait while we load your content...