Synthesis of ferrocene-functionalized monomers for biodegradable polymer formation†
Abstract
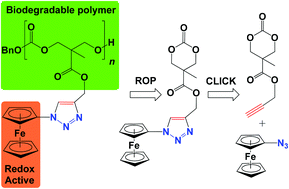
Cyclic carbonate and δ-valerolactone substrates functionalized with ferrocene were synthesized via alkyne–azide “click” cycloaddition. The cyclic carbonates were polymerized using 1,8-diazabicycloundec-7-ene, 1-(3,5-bis(trifluoromethyl)phenyl)-3-cyclohexylthiourea, and benzyl alcohol. The resulting polymers were characterized by GPC, NMR spectroscopy, and cyclic voltammetry studies. It was found that polycarbonate molecular weights fall in the range of 4.1–5.2 × 103 g mol−1 with polydispersities as low as 1.26. Electrochemistry studies allowed us to identify the monomer/polymer pair with the most desirable redox potential for biological studies.

 Please wait while we load your content...
Please wait while we load your content...