All-conjugated cationic copolythiophene “rod–rod” block copolyelectrolytes: synthesis, optical properties and solvent-dependent assembly†
Abstract
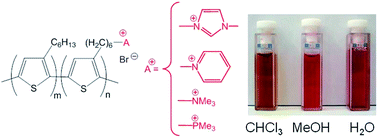
Amphiphilic diblock copolythiophenes were synthesised by an efficient two-step strategy. The diblock copolyelectrolytes were obtained via quasi-living Kumada catalyst-transfer polycondensation followed by quaternisation of the bromohexyl side chains of one of the monomer constituents into N-methylimidazolium, pyridinium, trimethylammonium or trimethylphosphonium units. The effect of the nature of the charged group on the thermal properties was investigated by Rapid Heat–Cool (RHC) calorimetry measurements. The solvent-driven assembly of these block copolyelectrolytes in chloroform (CHCl3), water, methanol (MeOH), water–MeOH mixtures and in subsequently prepared thin films was investigated using a combination of photoluminescence, scattering and microscopic techniques. The rigid rod-structure of the block copolyelectrolytes led to the formation of core–shell cylindrical or disc-like aggregates in solution, with features determined by the nature of the solvent. AFM studies revealed that the aggregates formed in solution can be transferred into thin films allowing for the reliable control of the self-organisation process and the resulting nanoscale architecture.

 Please wait while we load your content...
Please wait while we load your content...