A base–conjugate-acid pair for living/controlled ring-opening polymerization of trimethylene carbonate through hydrogen-bonding bifunctional synergistic catalysis
Abstract
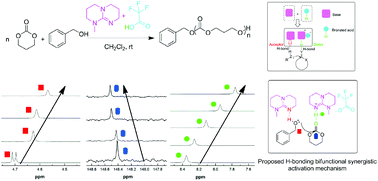
The ring-opening polymerization (ROP) of trimethylene carbonate (TMC) using a base–acid binary organocatalyst system of 7-methyl-1,5,7-triazabicyclo[4.4.0]dec-5-ene (MTBD) and Brønsted acid trifluoromethanesulfonic acid (TFA) with benzyl alcohol (BnOH) as the initiator has been investigated. The MTBD and its conjugate-acid pair H-bonding bifunctional synergistic activation mechanism was demonstrated by NMR measurements, and the living/controlled nature of MTBD/TFA catalyzed ROP of TMC was confirmed by the kinetic and chain extension experiments. The controlled ROP of TMC with the optimal molar ratio of MTBD/TFA = 2/1 proceeded to afford well-defined PTMC with narrow molecular weight distributions (Mw/Mn ∼ 1.1). The molecular weights determined by 1H NMR are in precise agreement with the theoretical values. 1H NMR, SEC, and MALDI-ToF MS measurements confirmed the structure of the obtained homopolymer PTMC. In addition, a diblock copolymer consisting of poly(trimethylene carbonate) and poly(L-lactide) (PTMC-b-PLLA) was synthesized successfully by the MTBD/TFA binary catalyst system.


 Please wait while we load your content...
Please wait while we load your content...