Activities comparison of Schiff base zinc and tri-zinc complexes for alternating copolymerization of CO2 and epoxides
Abstract
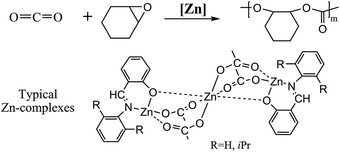
A series of salicylaldiminato-zinc and -tri-zinc complexes containing various Schiff base ligands were prepared using quick methods and then well identified by full characterization. All zinc complexes were examined as catalysts for the copolymerization of CO2 and cyclohexene oxide. Many factors such as electron-donating or -withdrawing substituents on the benzene rings of the ligands, as well as chloride anions or acetate groups bound to the zinc centers, are found to greatly influence on the insertion of CO2 and ring-opening of epoxides. Tri-metallic complexes 2a and 2b exhibited excellent catalytic activities, and thus the copolymerization conditions such as temperature, pressure and reaction time were optimized. The molecular weights of the resulting copolymers determined by gel permeation chromatography display a bimodal distribution with relatively wide polydispersities. The results of the investigation indicate that the catalytic activities of zinc complexes are highly dependent on the electronic density and steric environment around the Zn metal centers.

 Please wait while we load your content...
Please wait while we load your content...